Finding new host-directed
antiviral drugs
Our strategy to identify new host-direct antiviral drugs is based on two experimental routes:
In the Expedited Track, broad spectrum antiviral drugs for repurposing will be selected followed by in vitro and in vivo efficacy testing, and a clinical trial as a proof of concept.
The Elaborated Track include complex and concerted experimental schemes employing machine learning, high density cell arrays, primary cell cultures and others.
We will provide a sustainable pandemic preparedness pipeline ready-to-operate to identify the appropriate treatment against the emerging virus at the early time of a new outbreak. These studies will be conducted in several work packages as depicted here:
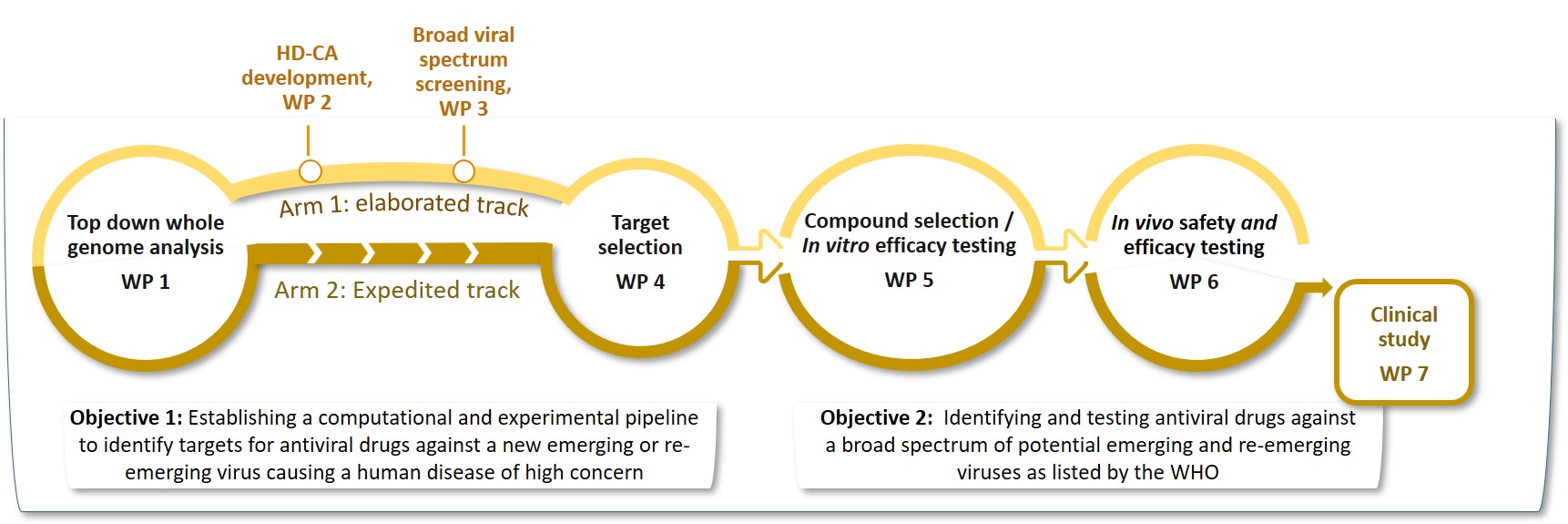
The Elaborated Track
In WP 1, the consortium will (i) perform two new genome-wide knockout screens to identify human genes that regulate Nipah and Lassa infected cells and (ii) combine the results with data of more than 100 publicly available screens and the literature into a machine learning pipeline. This will generate a list of about 2,000 genes coding for host dependency factors (HDF) and host restriction factors (HRF) comprising specific and common HD/HR factors across the investigated virus entities.
In WP 2, high density cell arrays will be constructed to target the genes in a mid-scale screening which will be performed in WP 3. Hits will be further investigated in WP 4 to identify the best candidate drug targets and combinations of drug targets. In WP 5, drugs for these targets will be selected and their efficacy tested in cell lines, primary cells and human relevant organotypic models. The best resulting drugs will be tested in animal models in WP 6.
The Expedited track
In WP 1 and WP 4, drug targets will be identified based on the literature and data integration by machine learning using HDF/HRF data of the existing > 100 screens, similar as for the Elaborated Track. To be fast, WP 2 and 3 are skipped in this Arm. The selected targets will be passed to WP 5 for selecting drugs for repurposing, followed by efficacy testing of the drugs. The best performing drugs will be tested in animal models in WP 6 followed by a clinical study in WP 7 validating the drug with the best efficacy.
This strategy will pave the way to an emergency response or a new pandemic, respectively, to any newly emerging virus causing a yet unknown disease in the future.
